Kathy Van Den Houwe
Scientific Institute of Public Health, Belgium
Title: A safety evaluation of printed paper and board contaminants: Photo-initiators as a case study
Biography
Biography: Kathy Van Den Houwe
Abstract
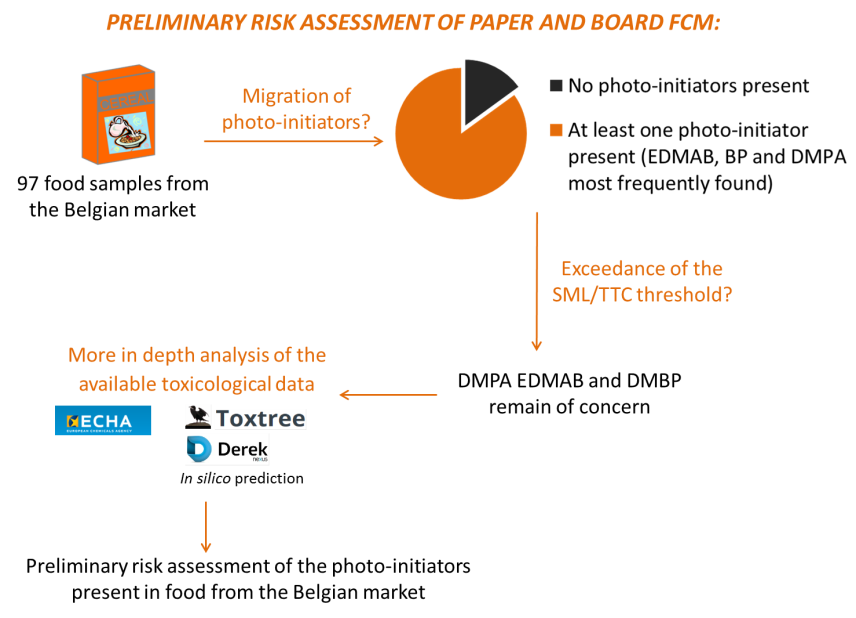
Consumers are exposed to a range of substances that can damage their health. Until recently, food packaging as a source of contaminants has received little attention despite its ubiquitous use. Several food crises at the end of the previous decade highlighted the need for more information about compounds used in food contact materials (FCM). At present, there is no specific European legislation for the use of printing inks on FCM. However, the Council of Europe has established general recommendations for various non-harmonized materials containing inventory lists of starting substances, together with their toxicological evaluation - whenever this information is available. In practice, for thousands of substances used in FCM, no safety evaluation has been performed at the European level. Specific classes of substances are photo-initiators that are widely used in UV-cured inks. First, a Belgian market survey was conducted in order to investigate the presence of photo-initiators in dry food. 97 dry foods were analyzed as marketed. At least one photo-initiator was found in 85% of the food samples and the photo-initiators that were most frequently found were benzophenone, 2, 2-dimethoxy-2-phenyl acetophenone (DMPA) and ethyl-4-dimethylaminobenzoate (EDMAB). Next, the potential genotoxicity of these substances was evaluated using a battery of in silico assays. Finally, a preliminary risk assessment was performed. For the evaluated substances, the concentrations in the food were below their Specific Migration Limit (SML). For the unlisted substances, the threshold of toxicological concern (TTC) approach was applied. The estimated exposure of the unlisted substances, EDMAB, DMPA and 4-dimethylaminobenzophenone, exceeded the human exposure threshold determined by the TTC-concept. For these substances, a more in-depth risk assessment was performed based on the toxicological information collected from the database of the European Chemicals Agency (ECHA). The current study illustrates how the TTC approach can be used to perform a preliminary risk assessment of substances migrating from FCM. However, the safety evaluation of these migrants remains challenging.